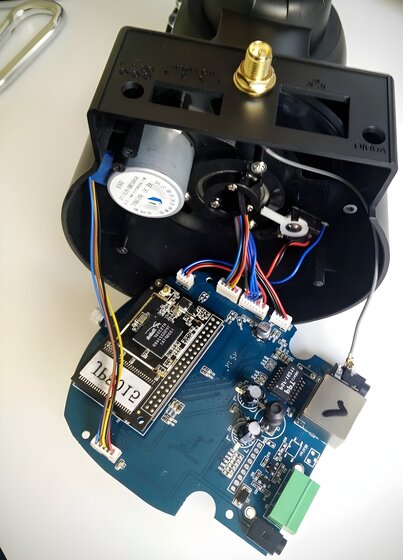
Audit
-

-

-
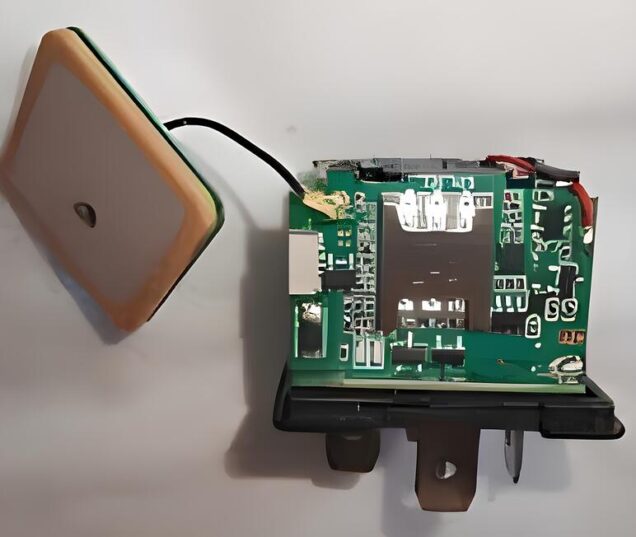
-

-

-

-

-
ISO13485 Medical Device Second Party Audit & Consulting
What is ISO 13485? ISO 13485 is the internationally recognized Quality Management System (QMS) standard specifically designed for the medical device industry. It provides a comprehensive framework for organizations involved in the design, production, installation, and servicing of medical devices, ensuring that products consistently meet customer and regulatory requirements. The standard emphasizes risk management, product […]
-

AS9100 Aerospace Parts Manufacturer Audit
What is AS9100? AS9100 is the internationally recognized Quality Management System (QMS) standard for the aerospace, aviation, and defense industries. It builds upon the ISO 9001 framework, incorporating additional requirements specific to the stringent demands of these sectors, such as risk management, configuration control, and product traceability. Developed by the International Aerospace Quality Group (IAQG), […]


